|
MST-READI recommends the use of a ‘true’
experimental design whenever possible so that you have the best chance
to determine causality, i.e., to determine that the technology being
evaluated is the cause of the outcomes being assessed.
The pre-post test control
group design is commonly used to answer questions addressed in
summative evaluation research. In this design all participants take a
pretest, then a subset of participants receive the new training
(experimental group) while the remainder receive traditional training
(control group), and finally all participants take a post test. In
general, pretest-posttest designs are the preferred method to compare
participant groups and measure the degree of change occurring as a
result of training. Control groups are intended to provide a group
against which to compare the experimental group to see examine changes
related to the training.
Regardless of the specific design you
choose, conducting an experiment will typically follow the sequence of
events below.
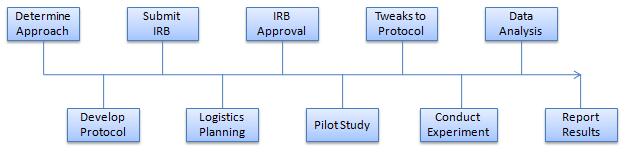
1. Determine Approach
–As mentioned earlier, a true experiment is recommended, however may not
always be possible. The evaluation team will gather necessary
information to determine a true experiment is feasible.
2. Develop Protocol
- For any design, an experimental plan, or protocol, should be developed
defining important components of the research and data collection
procedures.
3. Submit IRB
- Once the protocol is finalized, it should be submitted to IRB if
review and approval is required.
4. Logistics Planning
- While the protocol is being reviewed, the evaluation team should work
with the facility to support the logistics of the implementing
experimental procedures.
5. IRB Approval
- Data collection cannot begin until IRB approval is obtained.
6. Pilot Study
- Data collection is time and resource intensive; therefore, once
preliminary activities are complete and the protocol is approved by IRB,
a pilot study should be conducted.
7. Tweaks to Protocol
- The goal of the pilot study is to test experimental materials and
procedures and make recommendations for tweaks or modifications to
facilitate effective, efficient, and valid data collection.
8. Conduct Experiment
- After tweaks to the protocol are made, the experimental data
collection should be scheduled and may begin.
9. Analyze Data
- Once all data is collected it will be analyzed
10. Report Results
- Research results will be documented for presentation / reporting |